The first pathways we will discuss in this series are the Mitogen-Activated Protein Kinases (MAP Kinases, MAPKs). This type of signaling protein is common in all multicellular eukaryotes, and is potentially the most widely utilized signaling mechanism on the planet. In humans there are three families of MAPKs, the Extracellular Signal-Regulated Kinases (ERK), c-Jun N-terminal Kinases (JNK), and p38 MAP Kinases (p38), with each family providing its name to the larger signaling pathway in which it participates. The specifics of each will be discussed in their subsequent articles which will be linked here once they have been published:
ERK – coming soon
JNK – coming soon
p38 – coming soon
Kinases on Kinases on Kinases
The core function of a MAPK is to phosphorylate transcription factor (TF) substrates to activate (or sometimes inhibit) gene expression. Proteins with such close functional proximity to the genome must be regulated very strictly, as relatively small changes in the activity of a MAPK can have large impacts on transcription. Once these programs are turned on at the transcription level it can take hours to days for that effect to resolve (due to the half-life of eukaryotic mRNAs). For this reason, MAPKs are typically synthesized in an inactive state which must be relieved by one or more phosphorylation events before becoming fully functional. It is also important to note that a phosphatase is required to remove the phosphate groups and terminate the MAPK signal, the significance of which will be discussed later.
The job of phosphorylating the MAPKs is left to a creatively named group of proteins: the MAPK Kinases (MAP2K). Each individual MAPK pathway has multiple associated MAP2Ks which generally show low cross-reactivity outside the pathway. If the title of this section didn’t already spoil the ending, you may be surprised to find out that the MAP2Ks also require phosphorylation to become functional, and this is dependent on the MAPK Kinase Kinases (MAP3Ks). The MAP3Ks can be considered the entry point of a signal into the pathway, and associate closely with receptor proteins, adapters (such as MAP4Ks), and some second messengers/environmental factors. A depiction of this can be found in Figure 1.
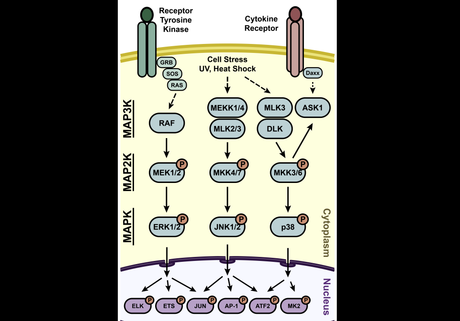 Figure 1 – Overview of the MAPK pathways with idealized inputs activating the MAP3Ks and initiating the activation of downstream kinases.
Figure 1 – Overview of the MAPK pathways with idealized inputs activating the MAP3Ks and initiating the activation of downstream kinases.
The arrangement of the pathways in this multi-tiered architecture serves some important purposes. More steps between the signal and the nucleus means more chances for regulation along the way. Every step in the pathway represents a balance between kinase and phosphatase activity, such that the signal should be self-limiting in the absence of continued stimulation. This allows for rather precise timing of signal termination which is critical during development, and prevents aberrant activation after low-level stimulation. The use of multiple MAP3Ks and MAP2Ks per pathway also shows how the conserved pathways can be used in slightly different ways depending on the broader context inside the cell.
We will see the iterations on this general structure in much more detail in subsequent articles, but the cascades presented in Figure 1 show the general conserved structure of the pathway. All pathway diagrams are necessarily simplified (usually to illustrate a specific experimental result), so it is difficult to appreciate the interplay between pathways and the other factors that regulate each step. As we begin to add details to the individual pathways take note of how much more crowded the picture becomes and how infrequently a specific protein will only have one input and output as shown for many in Figure 1.
It is also important to note that once the signal reaches the nucleus, many of the TF targets of the MAPKs are shared between two or more pathways. While at first this might seem like a problem but the cell solves this by typically only expressing one of these pathways at a time. That being said, the interplay between the MAPK pathways (and the role they play in non-MAPK pathways) is important to understand and will receive its own discussion.
A Round of Introductions
Now that we are familiar with the general structure of the MAPK pathways, let’s briefly meet the specific members of the family we’ll get to know over the next few articles.
ERK (Extracellular Signal-Regulated Kinases)
Structure and Activation:
Two canonical ERKs are found in humans: ERK1 (MAPK3) and ERK2 (MAPK1). Activation of ERK typically involves an extracellular signal, such as growth factors or mitogens, triggering the activation of a receptor tyrosine kinase (RTK). This, ultimately leading to the phosphorylation and activation of ERK which must translocate to the nucleus to interact with its TF partners (including Ets, Elk, and c-Jun/c-Fos) to modulate their activity.
Functions:
ERKs are primarily associated with cell proliferation, differentiation, and survival. They regulate the expression of genes involved in cell cycle progression and are often involved in promoting cell growth and tissue repair. ERK signaling is commonly associated with oncogenesis when dysregulated, with this signaling pathway being aberrantly up-regulated in about one-third of all human cancers. However, the ubiquity of this pathway means that few therapeutic interventions are specific enough to be considered viable options.
JNK (c-Jun N-terminal Kinases)
Structure and Activation:
JNKs are a group of three protein kinases: JNK1 (MAPK8), JNK2 (MAPK9), and JNK3 (MAPK10). JNKs are activated in response to various stressors, including UV radiation, inflammatory cytokines, and cellular damage. Active JNK will either translocate to the nucleus and activate TFs (including AP-1, c-Jun, p53, and ATF) or alternatively act on mitochondrial proteins (such as Bcl2) to induce Caspase-mediated apoptosis.
Functions:
JNKs were previously referred to as Stress-Activated Protein Kinases (SAPKs). They play key roles in stress responses, apoptosis, and immune regulation. JNK signaling can lead to the activation of transcription factors involved in cell death, inflammation, and cellular adaptation to stress. While it may seem that JNK activation would be beneficial in cancer (to induce apoptosis of cancer cells), it has actually been shown that activated JNKs will interact with other signaling pathways in some contexts to promote cancer cell survival. These features and others make it a complicated pathway to drug.
p38 MAP Kinases
Structure and Activation:
The p38 MAP kinase family consists of four isoforms: p38α (MAPK14), p38β (MAPK11), p38γ (MAPK12), and p38δ (MAPK13). Similar to JNKs, p38 kinases are activated by stress-related stimuli and proinflammatory cytokines, and this similarity extends to the TF substrates which are regulated by nuclear p38. Some differences do exist, however p38 is the least understood of the MAPKs so most of what we know comes from disease models.
Functions:
p38 MAP kinases are involved in various cellular responses to stress (including reactive oxygen species), inflammation, and cytokine signaling. They play critical roles in regulating immune responses, inflammatory processes, and cellular adaptation to environmental challenges. Dysregulation of p38 signaling is associated with inflammatory diseases, and it plays known roles in breast cancer.
Homology Within the MAPK Proteins
As members of the same class of proteins, it makes sense that the various MAPKs would share significant homology, especially as it relates to their catalytic domains which mediate the phosphorylation of serine and threonine residues. Figure 2 shows a portion of the amino acid (AA) sequences for the major human MAPKs aligned using Clustal Omega. Significant portions of homology can be noted (as indicated by asterisks below the sequences), and many non-identical residues show conserved AA properties (two dots = very similar, one dot = somewhat similar). The high homology punctuated by regions of low to no homology is common in the members of protein families, demonstrating how each took a slightly different evolutionary trajectory and evolved alongside their upstream and downstream targets.
 Figure 2 – Clustal Omega alignment of various human MPAK proteins (one representative isoform for each). AA # indicates ending residue for the indicated line. Asterisks indicate identical AA. One or two dots indicate low and partial residue similarity (by residue type and charge). Dashes in the sequence indicate gaps added for the purposes of alignment.
Figure 2 – Clustal Omega alignment of various human MPAK proteins (one representative isoform for each). AA # indicates ending residue for the indicated line. Asterisks indicate identical AA. One or two dots indicate low and partial residue similarity (by residue type and charge). Dashes in the sequence indicate gaps added for the purposes of alignment.
Into the Weeds We Go!
Now that we have surveyed the general topography of the MAPK pathways, we can begin exploring each in more detail. The articles, linked above and again below, will cover the members of each pathway, some specific examples of the pathway’s function in context (with a special focus on the immune system), and how each can become dysregulated in disease. These will be followed by a discussion of various surface receptors that activate MAPK signaling which will demonstrate that none of these signals act in a vacuum, and lead us into discussions of many other signaling systems in mammalian cells.
Thank you for reading! I hope you are enjoying this series so far, and please feel free to send in your suggestions for the future. Don’t forget to subscribe so you don’t miss the next one.
Stay curious!
The ERK Pathway – coming soon
The JNK Pathway – coming soon
The p38 Pathway – coming soon
Further Reading:
Reviews on MAPK function Plotnikov et al., Braicu et al., and Cargnello and Roux.
